bioMEMS IMPLANTABLE CHIPS
query duckduck go: : bioMEMS IMPLANTABLE CHIPS
images
https://duckduckgo.com/?q=bioMEMS+IMPLANTABLE+BIOCHIPS&t=h_&ia=images&iax=images
bioMEMS - wikipedia
https://en.wikipedia.org/wiki/Bio-MEMS
BioChips & BioMEMS report BioChips & BioMEMS (Bio Microelectromechanical Systems) - World Markets, Applications & Opportunities: Analysis & Forecasts to 2018
On A Chip – BioMEMS in Clinical and Point-Of-Care Applications
On A Chip - BioMEMS in Clinical and Point-Of-Care Applications - IEEE Life Sciences
Fully Integrated Biochip Platforms for Advanced Healthcare
Fully Integrated Biochip Platforms for Advanced Healthcare - PMC (nih.gov)
DARPA funded implantable biochip can potentially be used to deploy Moderna’s mRNA vaccine
in a bid to try to battle the ongoing Wuhan coronavirus (COVID-10) pandemic, the Defense Advanced Research Projects Agency (DARPA) is funding the development of an implantable biochip that could be deployed as soon as next year. The chip is said to be able to deploy an experimental new vaccine, developed jointly by Moderna and U.S government, that could change human DNA.
The chip and the vaccine built on a breakthrough made by then-Harvard University professor and eventual Moderna co-founder Derek Rossi in 2010. In his discovery, which the now-retired scientist says came about purely by accident, Rossi claimed that he found a way to “reprogram” messenger ribonucleic acid (mRNA) – the molecules that carry genetic instructions for cell development in the human body.
The promise provided by Rossi’s breakthrough was such that it allowed him to co-found Moderna with private equity firm Flagship Pioneering. The company then attracted almost half a billion dollars from the federal government to begin developing vaccines using the new technology.
Biochip could be used to deploy vaccine
In a preliminary report last July funded by the National Institute of Allergy and Infectious Diseases (NIAID) and the National Institutes of Health (NIH), Moderna’s mRNA-1273 vaccine was found to have “induced anti–SARS-CoV-2 immune responses in all participants and no trial-limiting safety concerns were identified.”
Despite this, however, one obstacle to the deployment of Moderna’s vaccine is the method of delivery. While Moderna is developing its own system, it’s unlikely to get Food and Drug Administration (FDA) approval any time soon. Enter Profusa, which is developing a nanoscale biochip that can detect symptoms of an infection.
Profusa’s biochip is made using a technology called “hydrogels” that were a product of the “In Vivo Nanoplatforms” (IVN) program that DARPA’s Biological Technologies Office (BTO) launched in 2014 to develop implantable nanotechnologies.
These hydrogels are soft, flexible nanomachines that are injected beneath the skin to perform monitoring. This hydrogel includes a specially engineered molecule that sends a fluorescent signal outside the body when it begins to fight infection. This signal can then be detected by a sensor attached to the skin that can then be sent to an app or even to a doctor’s website.
When it was first tested in 2018, this hydrogel was being used to measure glucose, oxygen and lactate levels. However, this past March, the company quietly announced that it was conducting a study to see if the technology could be used to detect respiratory diseases, including COVID-19.
THE MICROCHIP IS HERE: DARPA BIOCHIP TO ‘SAVE’ US FROM COVID CAN CONTROL HUMAN DNA
The Microchip Is HERE: DARPA Biochip To 'Save' Us From COVID Can Control Human DNA (wakingtimes.com)
Meet the Canadian hockey dad behind COVID-19 vaccine developer Moderna
Meet the Canadian hockey dad behind COVID-19 vaccine developer Moderna | National Post
A Military-Funded Biosensor Could Be the Future of Pandemic Detection
If it wins FDA approval next year, the two-part sensor could help spot new infections weeks before symptoms begin to show.
A Military-Funded Biosensor Could Be the Future of Pandemic Detection - Defense One
Why are pandemics so hard to stop? Often it’s because the disease moves faster than people can be tested for it. The Defense Department is helping to fund a new study to determine whether an under-the-skin biosensor can help trackers keep up — by detecting flu-like infections even before their symptoms begin to show. Its maker, Profusa, says the sensor is on track to try for FDA approval by early next year. ---
The announcement comes as the United States grapples with COVID-19, a respiratory illness that can present in flu-like symptoms such as coughing and shortness of breath. The military is taking a leading role in vaccine research, Joint Chiefs of Staff Chairman Gen. Mark Milley told reporters at the Pentagon on Monday. “Our military research labs are working feverishly around the horn here to try to come up with a vaccine. So we’ll see how that develops over the next couple of months,” Milley said. U.S. troops themselves are also at risk. A U.S. soldier in South Korea became the first U.S. service member to contract the virus, the Wall Street Journal reported in February.
Profusa and Partners Announce Initiation of Study to Measure Early Signs of Influenza Through Biosensor Technology
DARPA-Backed Study Leverages Profusa's Lumee® Oxygen Platform as Part of a Larger Effort to Speed Detection and Predict Disease Outbreaks
Despite the availability of antivirals and vaccines, influenza remains one of the greatest causes of illness and premature death worldwide. Seasonal influenza affects between 10% and 46% of the population each year, with mortality of up to approximately 12 deaths per 100,000 in developed countries.[1] During the 2009 H1N1 virus pandemic, many severe cases occurred in previously healthy young adults. With the entire worldwide population potentially at risk, the prevention and improved management of seasonal and pandemic influenza are of major importance.
"The potential significance of this new technology should not be underestimated, and Profusa is proud to be part of a joint effort funded by a DARPA, or Defense Advanced Research Projects Agency, award," said Sean Givens, head of government business for Profusa. "This is particularly exciting for Profusa as we look forward to leveraging learnings for future platform applications."
Profusa's Lumee Patch, a wireless reader that adheres to the skin and collects and reports tissue oxygen levels sensed by the Lumee Oxygen sensor to a mobile device for real-time data visualization, will be used in the clinical study.
Introduction to Applications of BioMems
Introduction to Applications of BioMems – uFluidic.com
So what exactly are bio MEMS? In general, bio MEMS are MEMS that are used in the medical field. For example, pacemakers and defibrillators use some of the same sensors that are found in cars smartphones, and cameras. These devices perform a medical or biological function therefore they are BioMEMS. Other types use biological components to perform a medical function or application. Applications for these devices exist in diagnostics and therapeutics detection and analysis drug delivery or cell culture. In addition, new emerging markets have made bio MEMS the largest and most diverse application of MEMS devices. ---
Sum-up
BioMEMS are systems that use MEMS, or biomolecular components to sense, analyze, measure, or actuate. This is a brief overview of some of the thousands of BioMEMS being used in the medical field today and others in development and testing. The MEMS market for medical applications is currently approximately two point 1 billion dollars and is projected to grow at double-digit rates for the next decade. With the current market of 2.1 billion, we think it's safe to say that bio MEMS devices are already impacting every aspect
Recent Advances in Implantable and BioMEMS Electronics and Their Applications with the Human Microbiome
A brief history of implantable electronics
Implantable electronics have been around for over six decades, with the first application dating back to just a few years after the transistor was invented and frequency-modulated (FM) radio signals were established. In 1959, Mackay Noller and others developed and tested radio transmitters that communicated physiological information from within the human body, primarily the gastrointestinal tract. Shortly after that, other researchers incorporated biotelemetry units to measure and report information on the human biome in a variety of body cavities without disturbing physiological parameters and optimizing sensing techniques and data transmission. The first major generation of implantable (and swallow-able) biotelemetry in the 1960s reported cardiovascular (electrocardiogram [ECG], blood pressure, and flow), respiratory (oxygen and temperature), neurophysiological (nerve activity), and gastrointestinal (pressure, pH, and temperature) parameters. Figure 1 shows a couple of telemetry circuits from the 1960s.
By the late 1970s, biotelemetry technology had progressed at rocket speed with more advanced and long-term applications ranging from pacemakers, nerve stimulators, insulin dispensers, and even sports medicine applications such as intra-cranial pressure (ICP) monitoring. Figure 2 shows a battery-less system that radiates a frequency proportional to surrounding cranial pressure used for head-trauma analysis.
MEMS technology and its applications in the microbiome
Organ-on-a-chip is a relatively new technology utilizing a combination of microfluidics, biomedical microelectromechanical systems (or bioMEMS), and biomaterial to mimic and simulate multi-level organ systems on a lab bench. This allows for real in-vitro studies such as drug testing or disease research while also developing useful implantable function-assistant devices. Brain, liver, heart, kidney, lung, and intestine functions have all been successfully implemented on a chip platform and have integrated various types of electronics.
Many organ-on-a-chip devices incorporate microsensors (or MEMS sensors) that assist with transducing things like pH, temperature, pressure/force, acceleration, humidity, sound/vibration, magnetic fields, and other biological or chemical parameters into an electrical signal. These microsensors almost always include a mechanical element (such as a diaphragm or cantilever beam) integrated with other microelectronics in a very small form factor but generally require some sort of signal processing and calibration or compensation to interface with it. Capacitive sensors are one of the most common sensing techniques used in MEMS devices and generally have a very small range. There are many ways to convert a variable capacitance to a measurable signal such as an oscillator or bridge. See Figure 3 for some examples of these analog interfaces.
Challenges associated with implanting electronics and designing bio-electromechanics
There are many difficulties associated with implanting or interfacing an electronics device with a human body, including minimizing size, weight, and power consumption while protecting the circuitry from the harsh and humid environment. And above all, the device must be safe and reliable.
Let’s start with size. How do you make something as small as possible without sacrificing performance? It helps to have a design process that allows mechanical and electrical engineers to work very closely together on optimizing package and mechanical layout, and CAD systems today have progressed by leaps and bounds over historical design methodologies in allowing complete visualization before any hardware is even produced.
But reliability is a big one, too. Many implanted and bioMEMS electronics are hermetically sealed by a process called “potting,” which creates a hardened, gelatinous barrier between the components and the enclosure. And depending on the classification rating of the medical device (implantables are generally Class III, the highest risk class), certain IEC standards will require it to be tested against rigorous safety protocols and require backup functions and redundancies.
And lastly, power optimization (particularly for implantables) has been a constant battle for engineers. We’d like to think we can tap into Iron Man’s Arc Reactor core power source, but instead, we use small batteries, supercapacitors, energy-harvesting techniques, and highly optimized low-power features of components. In-vivo energy harvesting (IVEH) has been a recent area of exploration that utilizes piezoelectric and triboelectric effects, endocochlear potential, biofuel cells, and light to provide ways of trickle charging batteries or capacitors. In 2014, a research team from Korea developed a pacemaker that is powered completely by flexible piezoelectric material, called a “nanogenerator.” See Figure 4 for an illustration of the pacemaker’s power path.
Examples of implantables, microbiome-on-a-chip, and bioMEMS products
We’ve discussed the evolution of microsensors/microelectronics and how they’ve been used in the human microbiome and simulated organs, but now let’s see them in action.
French company Biomillenia, in combination with QIAGEN’s microbial bioinformatics platform, has developed a droplet-based microfluidics chip platform used for culturing and analyzing microbes and bacterial species at an unparalleled level. Their platform can screen up to 100 million microbes in three days, whereas traditional methods would take up to three years while requiring a much larger volume for analysis.
At Tufts University, Fiorenzo Omenetto has studied how to use silk with an LED array as a dissolvable, implantable device to indicate the concentration in the blood of biomarkers, like insulin. The idea is for the silk to hold the LED/transistor array in place, then use antibodies or enzymes to detect biomarkers or disease markers, and then eventually dissolve and leave behind the silicon electronics without the need of surgery for removal.
The CardioMEMS HF System (seen in Figure 5) by Abbott is an implantable device utilizing MEMS technology that proactively monitors blood pressure and wirelessly transmits to a base unit for patient readout, allowing for reduced heart failures and indications before the symptoms even appear. It also opens up options for telemedicine and personalization of patient treatment plans based on the measurements produced by the device.
Implantable Insulin Pump Now Possible With MEMs Technology
Implantable Insulin Pump Now Possible With MEMs Technology | CytoFluidix
MEMS TECHNOLOGY
Would you use an implantable insulin Pump? Debiotech is a medical device company, based in Switzerland. The development of innovative medical tech is their main business and, since its inception, the company designed many award winning products.
The company brought to market electronic infusion systems, drug delivery devices, microsystems, imaging devices and nano pumps. But, what makes Debiotech unique is the vast experience they have in micromechanics, nanotech, microelectromechanical systems (MEMs) and implantables.
Together with the French/Italian manufacturer STMicroelectronics, Debiotech created a miniaturized insulin a pump that will change the lives of diabetics around the world.
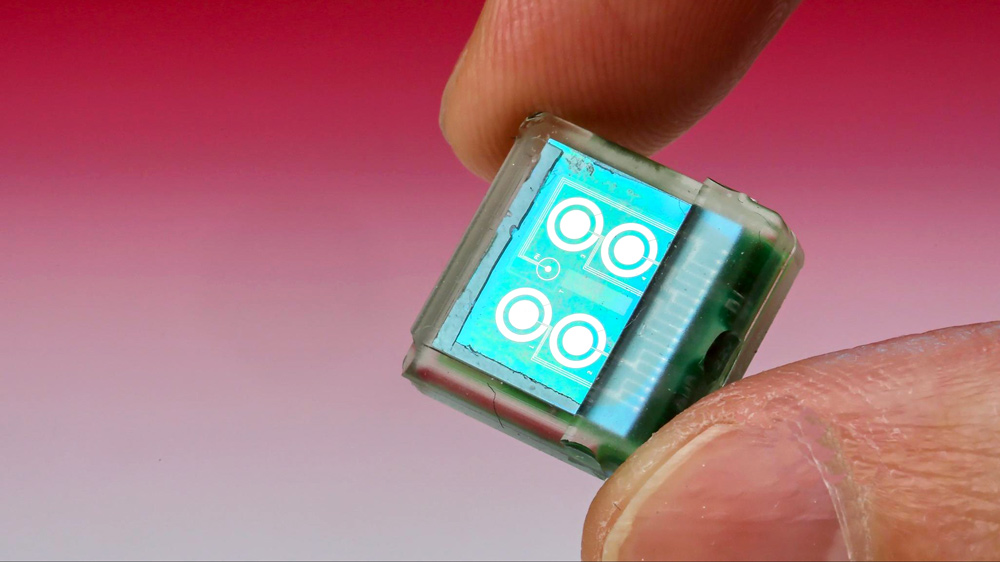
Implantable Bio-MEMS for the treatment of Cancer
Implantable Bio-MEMS for the treatment of Cancer | Semantic Scholar
Treatment of cancer has invoked technological forays that have been vying with each other and which were often supplanted by researchers with fresh and innovative approaches. Notable among these novel approaches is the Micro-Electro-Mechanical System (MEMS) technology, as it has established accomplishments in a variety of industrial areas. We are focusing on the therapeutic applicability of MEMS in biomedical arena, where they are called as Bio-Micro-Electro-Mechanical System (BioMEMS). We are presenting an analysis of the design, principle and performance of various BioMEMS devices and discuss the niche of their efficacy. We are emphasizing on the devices which could be implanted to deliver a single drug or a mixture of drugs in microlitre / nanolitre doses in a controlled timeframe. These BioMEMS are not meant to detect or diagnose but they offer a potential to treat malignancy and prevent the exposure of healthy cells from the severity of chemicals that could harm them.
Small-scale systems for in vivo drug delivery
[PDF] Small-scale systems for in vivo drug delivery | Semantic Scholar
Recent developments in the application of micro- and nanosystems for drug administration include a diverse range of new materials and methods. New approaches include the on-demand activation of molecular interactions, novel diffusion-controlled delivery devices, nanostructured 'smart' surfaces and materials, and prospects for coupling drug delivery to sensors and implants. Micro- and nanotechnologies are enabling the design of novel methods such as radio-frequency addressing of individual molecules or the suppression of immune response to a release device. Current challenges include the need to balance the small scale of the devices with the quantities of drugs that are clinically necessary, the requirement for more stable sensor platforms, and the development of methods to evaluate these new materials and devices for safety and efficacy.
NANO/MICROSCALE TECHNOLOGIES FOR DRUG DELIVERY
NANO/MICROSCALE TECHNOLOGIES FOR DRUG DELIVERY | Semantic Scholar